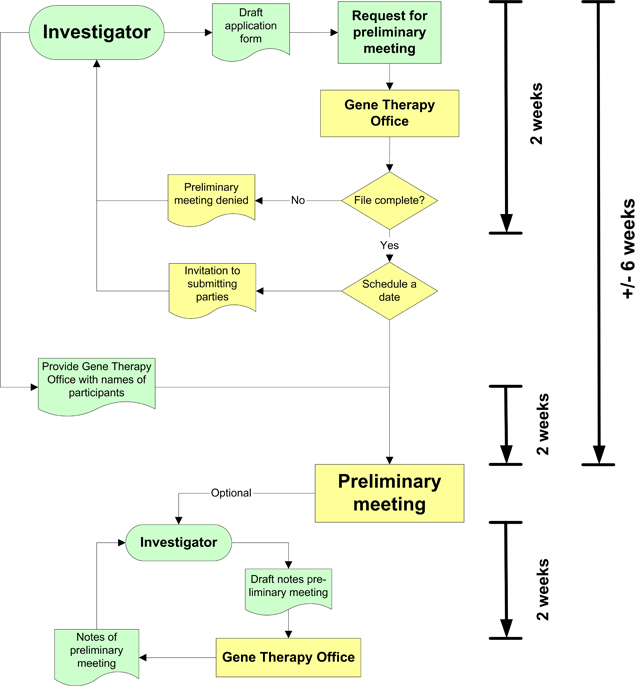
The Gene Therapy Office is responsible for organising the joint preliminary meeting. In principle, the preliminary meeting will take place within 6 weeks of the investigator’s request.
Together with his request for a preliminary meeting, the investigator must send all draft documents and any questions, digitally, to the Gene Therapy Office, who will ensure that all bodies participating in the preliminary meeting receive these documents. The preliminary meeting cannot take place until all participating bodies have agreed that the draft research file is complete. If one of the bodies does not agree then the preliminary meeting cannot go ahead in this form.
Once all the participating bodies have agreed, about 2 weeks after the investigator’s request for preliminary meeting, the Gene Therapy Office will propose a number of possible dates on which the preliminary meeting can take place. A date is then scheduled together with the investigator. The consultation lasts one and a half hours and usually takes place at a meeting location near Utrecht Central Station. The language spoken at the preliminary meeting is Dutch or English.
The preliminary meeting is always chaired by a member of CCMO (Central Committee on Research involving Human Subjects) or COGEM (Netherlands Commission on Genetic Modification). The investigator is given the opportunity to take notes of the preliminary meeting. Any notes must be sent to the Gene Therapy Office no later than 2 weeks after the preliminary meeting has taken place. These notes are then presented for verification to those who were present at the preliminary meeting. The final version of the notes is then sent to the investigator.
Diagram of Gene Therapy Office procedures concerning preliminary meeting